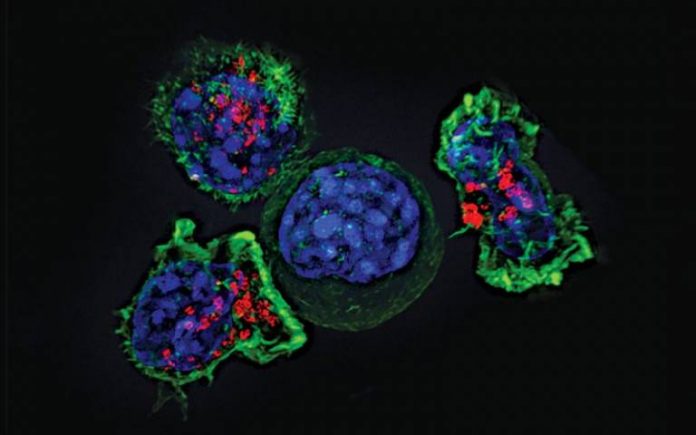
For this proof-of-principle study, researchers at the UCL Great Ormond Street Institute for Child Health (GOS ICH) and the UCL Cancer Institute modified the patient’s own T-cells (a type of immune cell), equipping them to recognise and kill neuroblastoma tumour cells.
Twelve children with relapsed or refractory (where the disease does not respond to treatment) neuroblastoma were treated as part of the Cancer Research UK-funded phase I clinical trial.
The research, published in Science Translational Medicine, is one of the first studies to demonstrate CAR T-cells achieving rapid regression against a solid cancer (non-blood cancer). Although the beneficial effects only lasted a short while, the study provides important evidence that this specific CAR T-cell treatment could be used as a future treatment for children with solid cancers.
Neuroblastoma is a rare type of cancer that mostly affects babies and young children and develops from specialised nerve cells (neuroblasts) left behind from a baby’s development in the womb.
Up to 100 children in the UK are diagnosed with neuroblastoma each year. Current treatment for children with an aggressive type of neuroblastoma includes surgical removal, chemotherapy with stem-cell transplant, radiotherapy and antibody therapy. Despite this intensive treatment long-term survival is between 50-60 per cent.
In CAR T-cell therapy, a type of immunotherapy, T-cells are engineered to contain a molecule called a chimeric antigen receptor (CAR) on their surface which can specifically recognise cancerous cells.
For this study the patients’ own T-cells were modified with a CAR to target the GD2 surface protein, which is highly abundant on almost all neuroblastoma cells, but found at very low levels in healthy cells.
Researchers found that when using a sufficient dose* of the modified CAR T-cells, this treatment induced rapid reduction in tumour size in some of the patients treated. These effects were transient. Importantly, in all patients the CAR T-cells did not cause any harmful side effects in healthy tissues that express the GD2 molecule.
Lead author, Dr Karin Straathof, research group leader at UCL GOS ICH and Consultant Paediatric Oncologist at Great Ormond Street Hospital NHS Trust said: “It’s encouraging to see the anti-tumour activity induced by these modified T-cells in some of the patients on this study.
“While the anti-tumour activity seen was only transient, it provides an important proof-of-principle that CAR T-cells directed at the GD2 molecule could be used against solid cancers in children.
“New treatments are needed for high-risk neuroblastoma and with more research we hope to develop this further into a treatment that results in lasting responses and increases the number of patients that can be cured.”
Senior author, Dr Martin Pule (UCL Cancer Institute) said: “Targeting of solid cancers by CAR T-cells is dependent on their infiltration and expansion within the tumour microenvironment, and thus far fewer clinical responses have been reported.
“The rapid regression in neuroblastoma cells is promising, particularly as this activity was observed in the absence of neurotoxicity which occurs with antibody-based approaches that target GD2.”
Dr Pule added: “Targeting neuroblastoma with GD2 CAR T-cells appears to be a valid and safe strategy but requires further modification to promote CAR T-cell longevity.”
Dr Sue Brook, medical advisor at Cancer Research UK, said: “Children who have hard to treat cancers like neuroblastoma have limited treatment options open to them, especially when the cancer returns.
“The early results for the GD2 CAR-T treatment look promising, especially due to the initial safety data. However more work is needed on making the response last longer, and we are looking forward to seeing the next steps in its development.”
The research team are preparing for their next clinical study in collaboration with Autolus, a clinical-stage biopharmaceutical company developing next-generation, programmed T-cell therapies for the treatment of cancer. This study will evaluate AUTO6NG, which builds on this approach utilising the same GD2 CAR alongside additional programming modules designed to enhance efficacy and persistence.
* To establish the minimal effective dose, escalating doses of GD2-directed CAR T-cells were used. The first group of patients was given a low dose, the second group a higher dose, and a third group a higher dose still. Each patient received one dose only. Researchers found that a minimal cell dose of 108/m2** was needed for the CAR T-cells to divide and become activated once administered to the patient.
** The dose is expressed per square metre surface area as this is a paediatric study and that is how doses are calculated in children (or alternatively per kilogram of body weight). The cell dose is 108 per square metre body surface area or 100 million cells per square metre body surface area.